 消化系惡性腫瘤(結腸直腸癌)
消化系惡性腫瘤(結腸直腸癌)
 消化系惡性腫瘤(結腸直腸癌)
消化系惡性腫瘤(結腸直腸癌)
![]()
當大腸直腸癌有了轉移時,治療上常不能只僅依靠外科手術切除,需要佐以化學治療。雖然化學治療近年也有相當的進步,但大都需要以注射給藥,仍有不便之處,發展口服劑型的抗癌藥物成為今日藥界的顯學之一。
自1957年5-Fluorouracil (5-FU)出現後,大腸直腸癌的化學治療藥物基本上一直以為它骨幹,即使此單一藥物對大腸直腸癌的有效率只有兩成上下,加上其他藥物也有三至四成的反應率,但比起以安慰劑的療效仍令人注意。而這個藥物雖然出現半世紀之久,但對它在抗癌的藥理作用的瞭解,也不過是近十年來的事情。目前知道加上生物調控的藥物,例如leucovorin (LV) ,可加強了它的抗癌效價,治療成績也稍稍提升。所以迄今主要的化學藥物療程裡,都無法不使用5FU。不過使用上它需要靜脈注射,醫師病人無不企盼能有口服劑型的藥物出現。在美國有幾篇研究顯示無論是從使用上、心理層面、生活品質,轉移癌症患者的照顧上,口服抗癌藥物都得到八九成以上患者與醫師的肯定。臨床床上也少掉了一些靜脈注射的副作用,譬如感染、靜脈炎、血栓等等問題。
5-FU本身無法使用於口服,主要是因為它的生物活性無法預估。
希望口服劑型的效果,能保有原有效果,但更方便,少副作用,也能達到如長時間持續血液注射的療效。基於這樣的期望,目前有兩類相關於 口服劑型 fluoropyrimidine 的 藥物被發展出來。首先是對 5-FU 代謝的抑制劑,可影響腸道上主要代謝 5-FU的酵素 dihydropyrimidine dehydrogenase (DPD),增加 5-FU的吸量。另一類則是給於 fluoropyrimidine 的 prodrug,例如 capecitabine,它以原型,不活化的劑型吸收,進入體內,到達癌細胞處才被活化成有療效的 5-FU。
迄今已有多種口服Fluoropyrimidines 相關藥物,其中兩個為DPD抑制劑,另一個就是 capecitabine (Xeloda)。
|
N |
Response Rate (%) |
Median TTP (months) |
Median Survival (months) |
|
|
UFT |
||||
|
Pazdur and colleagues[8] |
||||
|
UFT/LV |
409 |
12 |
3.5 |
12.4 |
|
5-FU/LV |
407 |
15 |
3.8 |
13.4 |
|
P value |
- |
NS |
.011 |
NS |
|
Hazard ratio* |
- |
.78 |
1.22 |
1.07 |
|
Carmichael and colleagues[9] |
||||
|
UFT/LV |
195 |
11 |
3.4 |
12.2 |
|
5-FU/LV† |
185 |
9 |
3.3 |
11.9 |
|
P value |
- |
NS |
NS |
NS |
|
Hazard ratio* |
- |
1.20 |
1.06 |
.86 |
|
Eniluracil/Oral 5-FU |
||||
|
Van Cutsem and colleagues[10] |
||||
|
Eniluracil/oral 5-FU |
268 |
12 |
NR |
10.9 |
|
5-FU/LV |
263 |
14 |
NR |
14.6 |
|
P value |
- |
NS |
NR |
NR |
|
Hazard ratio* |
- |
NR |
1.20 |
1.30 |
|
Levin and colleagues[11] |
||||
|
Eniluracil/oral 5-FU |
485 |
12 |
4.6‡ |
13.2 |
|
5-FU/LV |
479 |
13 |
5.2‡ |
14.5 |
|
P value |
- |
NS |
.01 |
.31 |
|
Hazard ratio* |
- |
NR |
NR |
NR |
|
Capecitabine |
||||
|
Hoff and colleagues[12] |
||||
|
Capecitabine |
302 |
25 |
4.3 |
12.5 |
|
5-FU/LV |
303 |
16 |
4.7 |
13.3 |
|
P value |
- |
.005 |
.72 |
.974 |
|
Hazard ratio* |
- |
- |
1.03 |
1.00 |
|
Van Cutsem and colleagues[13] |
||||
|
Capecitabine |
301 |
27 |
5.2 |
13.2 |
|
5-FU/LV |
301 |
18 |
4.7 |
12.1 |
|
P value |
- |
.013 |
.65 |
.33 |
|
Hazard ratio* |
- |
- |
.96 |
.92 |
*Oral fluoropyrimidine vs 5-FU/LV
†Modified Mayo Clinic regimen
‡Progression-free survival
5-FU, 5-fluorouracil; LV, leucovorin; TTP, time to progression; UFT, uracil plus tegafur; NS, not significant; NR, not reported
UFT has been evaluated extensively in combination with LV. In 2 randomized, phase 3 trials, UFT/LV was compared with intravenous bolus 5-FU/LV in patients with metastatic colorectal cancer.[8,9] The primary end point in both studies was overall survival. In both trials, UFT 100 mg/m2 and LV 25 mg to 30 mg were administered 3 times daily on days 1-28 of a 35-day cycle.
In 1 trial, UFT/LV was compared with LV 20 mg/m2 followed by 5-FU 425 mg/m2 as an intravenous bolus on days 1-5 of a 28-day cycle (standard Mayo Clinic regimen).[8] In the other, the comparator was the same dosing of 5-FU/LV, but administered on days 1-5 of a 35-day cycle (low-dose, modified Mayo Clinic regimen).[9]
Of these comparators, the standard Mayo Clinic regimen is the most relevant, being a commonly used schedule with proven efficacy. More importantly, it has also been the standard arm required by the US Food and Drug Administration in registration trials for new colorectal cancer therapies.
When compared with this standard regimen, UFT/LV was significantly inferior in terms of time to disease progression (TTP), and resulted in a 22% increase in the risk of disease progression compared with the control regimen.[9] The Kaplan-Meier (KM) curve showed that TTP was significantly inferior in the UFT/LV arm compared with the intravenous 5-FU/LV arm over the entire course of the study. This was confirmed by the hazard ratio (UFT/LV:5-FU/LV) of 1.22 (P < .01).
Although TTP was inferior with UFT/LV, overall median survival was similar in both treatment arms: 12.4 months in the investigational arm vs 13.4 months in the control arm. There was a trend toward lower response rates with UFT/LV (12% vs 15% with 5-FU/LV), although this was not statistically significant. In addition, UFT/LV was associated with a high rate of severe (grade 3/4) diarrhea (21%).
As a result of the significantly inferior efficacy of UFT/LV compared with the standard Mayo Clinic regimen, UFT/LV is not available for the treatment of colorectal cancer patients in the United States.
Another combination of a DPD inhibitor and an oral fluoropyrimidine, eniluracil plus oral 5-FU, has also been evaluated in 2 randomized, phase 3 clinical trials in patients with metastatic colorectal cancer.[10,11] Patients were randomized to receive either oral eniluracil 11.5 mg/m2 plus oral 5-FU 1.15 mg/m2 twice daily on days 1-28 of a 35-day cycle, or intravenous bolus 5-FU/LV (Mayo Clinic regimen).
Although well tolerated, eniluracil/5-FU demonstrated disappointing efficacy. As shown by the KM curves and the hazard ratios (eniluracil/5-FU:5-FU/LV), eniluracil/5-FU was statistically significantly inferior to intravenous bolus 5-FU/LV for TTP in one study[11] and overall survival in the other.[10] As a result of the poor efficacy demonstrated in phase 3 trials, the development of the eniluracil/5-FU combination has been abandoned.
The tumor-selective generation of 5-FU by capecitabine has been confirmed in a trial involving patients with colorectal cancer.[16] Patients were treated with capecitabine 1250 mg/m2 twice daily for 5-7 days before surgical resection of their primary tumor. Concentrations of 5-FU in primary tumor tissue were found to be 3.2-fold higher than in adjacent healthy tissue, and 21 times higher than in plasma. The presence of 5-FU outside of the tumor is thought to be due to its diffusion out of the cancer cell.
In total, 1207 patients were randomized to receive either capecitabine (603 patients), or Mayo Clinic 5-FU/LV (604 patients). The patients' baseline characteristics (gender, age, performance status, predominant metastatic site, and prior adjuvant treatment) were well balanced between the treatment groups.
The integrated analysis confirmed the results of the individual trials. The primary end point, tumor response rate, was higher in patients treated with capecitabine compared with patients receiving 5-FU/LV (26% vs 17%; P < .0002). This finding held up when response rates were analyzed by subpopulation (exposure to prior treatment, location, and number of metastatic sites.
TTP and overall survival in patients receiving capecitabine were equivalent to the 5-FU/LV group, with similar KM curves. Median TTP was 4.6 months with capecitabine compared with 4.7 months in the 5-FU/LV arm, and median overall survival was 12.9 vs 12.8 months, respectively.
Stomatitis, diarrhea, nausea, and alopecia were significantly less common with capecitabine than with 5-FU/LV (Figure 1). Vomiting and fatigue occurred at similar rates in both treatment groups. Palmar-plantar erythrodysasthesia (hand-foot syndrome), a skin inflammation that affects the palms of the hands and the soles of the feet, was significantly more frequent in patients receiving capecitabine, although this was not dose-limiting (Figure 1). Neutropenia, neutropenic fever/sepsis, and grade 3/4 stomatitis were significantly less likely to occur in patients receiving capecitabine compared with those treated with 5-FU/LV.
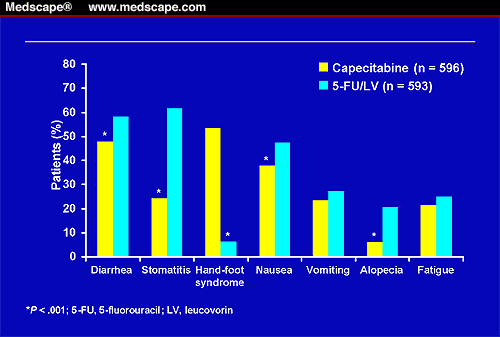
Figure 1. Most common treatment-related adverse events (all grades). Reprinted with permission from Hoff PM, Cassidy J, Schmoll H. The Evolution of Fluroropyrimidine Therapy: From Intravenous to Oral. Oncologist. 2001;6(suppl 4):3-11.
The incidence of treatment-related hospitalizations was significantly lower with capecitabine than with 5-FU/LV (12% vs 18%, respectively; P < .005). In the 5-FU/LV group, stomatitis and neutropenic fever/sepsis led to hospitalization in 21 and 17 patients, respectively, compared with only 1 patient each in the capecitabine arm. Hand-foot syndrome led to hospitalization of 2 patients in the capecitabine group.
Capecitabine treatment resulted also in a substantial reduction in medical resource use compared with 5-FU/LV.[19] This derived principally from the absence of hospital or infusion center visits for intravenous drug administration, fewer treatment-related hospitalizations, and the use of fewer supportive care drugs for treatment of side effects in patients receiving capecitabine.
Dose adjustment in patients with renal impairment at baseline. Analysis of subsets of patients treated in these studies revealed that the incidence of grade 3/4 adverse events was higher in patients with moderate renal impairment (calculated creatinine clearance 30 mL/minute to 50 mL/minute according to the formula of Cockcroft and Gault) than in those with mild impairment or normal renal function (calculated creatinine clearance > 50 mL/minute), regardless of the treatment arm. Consequently, dose reductions and treatment withdrawals were more frequent in patients with moderate renal impairment, independent of treatment.[20]
A separate pharmacokinetic study helped to explain this observation. It demonstrated that the area under the curve of a capecitabine metabolite and immediate 5-FU precursor, 5'-deoxy-fluorouridine, was increased by 35% in patients with moderate renal impairment compared with patients with normal renal function (C. Poole, unpublished data, 2001).
These are the data supporting the recommended dose reduction to 75% of the standard starting dose (ie, 950 mg/m2 twice daily) in patients with moderately impaired renal function at baseline, which can be expected to result in comparable systemic exposure as full-dose capecitabine in patients with normal renal function. Since age is an important variable in the calculated creatinine clearance, elderly patients will typically receive this lower starting dose. Indeed, even in the absence of supporting data, many clinicians had already adopted a lower starting dose (1000 mg/m2 twice daily) than the one used in these comparative studies.
Capecitabine is contraindicated in patients with severe renal impairment (calculated creatinine clearance < 30 mL/minute) (Table 2).[20]
|
Renal Impairment |
Calculated Creatinine Clearance (mL/minute) |
Starting Dose (mg/m2 twice daily) |
|
None |
> 80 |
1250 |
|
Mild |
51-80 |
1250 |
|
Moderate |
30-50 |
950 |
|
Severe |
< 30 |
Contraindicated |
The adverse events most commonly resulting in treatment modification were hand-foot syndrome and diarrhea in the capecitabine group, and stomatitis and diarrhea in the 5-FU/LV group.
The efficacy of capecitabine was not affected in patients requiring dose modification for adverse events. There was no increase in the risk of disease progression or death in patients requiring capecitabine dose modification compared with those who did not (hazard ratio = 0.97, not significant). The corresponding hazard ratio in the 5-FU/LV group was 1.12, representing a 12% increase in the risk of disease progression or death in patients requiring dose reduction (not significant).
Irinotecan, a semisynthetic camptothecin derivative that inhibits topoisomerase I, is approved for use in Europe and the United States in combination with 5-FU/LV as first-line therapy, and as a single agent for second-line therapy.[22]
Oxaliplatin is a third-generation platinum analog with activity and safety profiles that differ from those of other platinum derivatives, including cisplatin and carboplatin.[23] Oxaliplatin in combination with 5-FU/LV is approved in Europe as first-line therapy and a registration program for the use of oxaliplatin in combination with 5FU/LV following irinotecan/5-FU/LV failure is ongoing in the United States.
More recently, trials have been conducted to determine whether these new drugs are suitable for combination with oral fluoropyrimidines. Capecitabine, which has shown single-agent efficacy and a favorable safety profile as first-line treatment for colorectal cancer, is currently being evaluated in combination with these compounds.
Consequently, a number of phase 1 clinical trials have been conducted to establish the most appropriate first-line regimen of irinotecan plus capecitabine. These studies are evaluating a range of schedules, including continuous, flat dosing of capecitabine, a 14-day regimen of capecitabine, and irinotecan administered weekly or on days 1 and 8 every 3 weeks.
In an extended phase 1/2 study conducted in Germany, 37 patients with previously untreated, measurable metastatic colorectal cancer were treated with 49-day treatment cycles of capecitabine (1000 mg/m2 to 1250 mg/m2 twice daily on days 1-14 and 22-35) plus weekly irinotecan (70 mg/m2 or 80 mg/m2 on weeks 1-6).[27] Diarrhea and neutropenia were dose limiting. The recommended dose was identified as capecitabine 1000 mg/m2 twice daily plus irinotecan 70 mg/m2.
Of the 16 patients receiving the recommended dose, 4 patients experienced grade 3/4 diarrhea and 1 patient experienced grade 3/4 neutropenia. To date, 11 of 29 patients evaluable for response have achieved an objective response.
In another, ongoing, phase 1, dose-finding study, capecitabine (750 mg/m2 to 1250 mg/m2 twice daily on days 1-14 of a 21-day cycle) and irinotecan (200 mg/m2 to 350 mg/m2 on day 1 of the same cycle) have been administered at escalating doses to 27 patients with previously untreated metastatic colorectal cancer. An update of this study was presented at ECCO 11, in October 2001.[28]
The recommended schedule for planned phase 2 and pharmacokinetic studies was identified as irinotecan 250 mg/m2 on day 1 in combination with capecitabine 1000 mg/m2 twice daily on days 1-14 of a 21-day cycle. Preliminary efficacy data are promising, with an overall response rate of 48%, and tumor responses observed at all dose levels.
An open-label, phase 1, dose-finding study assessed capecitabine in combination with oxaliplatin in 23 patients with advanced/metastatic solid tumors, all of whom had failed standard therapeutic options.[31] A 21-day treatment cycle of oral capecitabine 1000 mg/m2 twice daily (days 1-14) and intravenous oxaliplatin 130 mg/m2 (day 1) was recommended for further evaluation.
The dose identified in the phase 1 dose-escalation study is now being evaluated in an international phase 2 study. The primary objective of this phase 2 study is to evaluate the overall response rate. Secondary objectives include time-related efficacy parameters and tolerability. Recruitment of 96 patients with previously untreated advanced/metastatic colorectal cancer is now complete and preliminary data were recently reported at ECCO 11.[32]
The combination achieved a response rate of 55%, with disease stabilization in 32% of patients. Median time to disease progression was 7.1 months.
Most adverse events with the capecitabine/oxaliplatin combination were gastrointestinal (vomiting, nausea, and diarrhea); hence, prophylactic 5HT3/corticosteroid antiemetic therapy is now recommended for oxaliplatin-based therapy. Grade 3/4 neutropenia and thrombocytopenia occurred in 8% and 5% of patients, respectively. Grade 3 hand-foot syndrome was not seen, and there has been a low incidence of grade 3/4 oxaliplatin-induced neurotoxicity to date, with only 1 case of laryngopharyngeal dysesthesia.
As a combination partner for irinotecan and oxaliplatin, capecitabine has shown activity and tolerability similar to combinations with infused 5-FU. Ongoing and planned trials will further explore the suitability of capecitabine as a combination partner.
Similarly, the addition of oxaliplatin to 5-FU/LV improved response rates and TTP in a first-line setting, and was also effective in 5-FU-resistant disease.[29,30,34] However, overall survival was not significantly improved by the addition of oxaliplatin to first-line 5-FU/LV.[29,30] The effect of oxaliplatin on survival may have been obscured by effective poststudy chemotherapy and the surgical removal of metastatic lesions in a high proportion of patients in the control arms, although this is merely conjecture. However, infused 5-FU/LV regimens seem to be better tolerated than bolus regimens, and irinotecan combination therapy resulted in a modest survival benefit with infused 5-FU/LV. The high proportion of patients crossing over to irinotecan in this study may explain why the gain was only modest.[24] These data suggest that crossover does affect the efficacy of first-line combination therapy; therefore, some oncologists support the use of sequential therapy.
Currently, there are no unequivocal arguments for using either combination or sequential chemotherapy in patients with colorectal cancer. Instead, treatment should be tailored for the individual patient, taking into account factors such as potential prognostic subgroups, toxicity profiles, and patient preferences.
A subgroup analysis of a phase 3, randomized study comparing first-line irinotecan/5-FU/LV with 5-FU/LV indicated that normal lactate dehydrogenase, hemoglobin, and bilirubin concentrations, good performance status, and normal white blood cell counts are major factors associated with improved survival and TTP.[35] Metastases at only 1 site were also associated with a better outcome with combination therapy.
Thus, patient subgroups unlikely to benefit from combination therapy who are at increased risk of developing toxicity can be identified (eg, those with poor performance status, abnormal liver function, or myelosuppression). These patients may be more appropriately treated with sequential administration of active therapies.
Prolonged infusion 5-FU is also effective during postoperative radiotherapy. A large, randomized trial conducted in 660 patients with International Union Against Cancer (UICC) stage II/III rectal cancer demonstrated that continuous infusion of 5-FU (225 mg/m2/day) for the 5-week duration of radiotherapy (total dose 45 Gy plus a 5.4-Gy boost) resulted in significantly improved overall survival (P = .005) and disease-free survival (P = .01) compared with bolus 5-FU administration.[36]
While the data clearly support the role of infusional 5-FU as a component of chemoradiotherapy for rectal cancer, the practical aspects of delivering the therapy are demanding for patients and medical resources. An additional reason to investigate whether capecitabine has the potential to replace infusional 5-FU, and simplify chemoradiation in patients with rectal cancer, lies in the fact that the activity of the enzyme TP, involved in the metabolism of capecitabine, is upregulated by radiotherapy in tumor cells, but not in normal cells.[37] Consistently, capecitabine/radiotherapy combination treatment has shown greater antitumor activity in colon and breast cancer xenografts compared with either agent alone, whereas 5-FU/radiotherapy combination treatment showed no clear additive effect.
On the basis of these observations, several phase 1 dose-finding studies of combinations of capecitabine and radiotherapy have been conducted and phase 2/3 trials are underway.
In one such study, conducted in Germany, 36 patients received radiotherapy (1.8 Gy to the pelvis, 5 times weekly; total dose 50.4 Gy, including a presacral boost of 5.4 Gy) plus capecitabine (250 mg/m2 to 1000 mg/m2) twice daily, with the first dose 2 hours before the daily radiotherapy treatment.[38] The maximum tolerated dose of capecitabine was 1000 mg/m2 twice daily; grade 3 hand-foot syndrome occurred in 2 of 6 patients treated at this dose level. There were no grade 4 toxicities, and the only other grade 3 adverse events were local skin toxicity, rash/itch, and diarrhea (1 patient each).
Unconfirmed data from 7 of 10 patients receiving neoadjuvant treatment indicate that the tumors demonstrated shrinkage in 5 patients, and that there was 1 pathologic complete response.
Another phase 1 study conducted in Australia included patients with locally advanced, potentially operable rectal cancer.[39] Treatment consisted of radiotherapy (1.8 Gy to the pelvis, 5 times weekly; total dose 50.4 Gy, including a presacral boost) and capecitabine (425 mg/m2 to 1000 mg/m2) twice daily, Monday to Friday, with the first dose administered 2 hours before radiotherapy. Surgery was scheduled 4-6 weeks after chemoradiation. To date, 15 patients have been enrolled, and the maximum tolerated dose of capecitabine has not yet been reached. So far, only 1 patient has experienced a grade 3/4 toxicity (cystitis).
Of the 15 evaluable patients, 14 have undergone surgical resection. There has been no operative mortality, and 6 minor postoperative complications. All patients have undergone curative resections, 2 of which revealed complete histologic responses. Currently, enrollment is continuing for patients receiving capecitabine at a dose of 1000 mg/m2 twice daily.
A randomized, phase 3 study is planned by the National Surgical Adjuvant Breast and Bowel Project (NSABP) (R-04) to compare capecitabine with infused 5-FU as combination partners for radiotherapy in rectal cancer. This study will involve patients with macroscopic, operable UICC stage II/III adenocarcinoma of the rectum, located < 12 cm from the anal verge.
Patients will be randomized to receive preoperative radiotherapy (45 Gy in 28 fractions, plus a 5.4-Gy boost for nonfixed tumors, and a 10.8-Gy boost for fixed tumors) plus capecitabine 825 mg/m2 twice daily or infused 5-FU 225 mg/m2/day, stratified according to age, gender, tumor stage, and tumor mobility.
Looking further ahead, biologic therapies appear promising, and treatment will become increasingly tailored to the individual, aided by prognostic and predictive markers, both biologic and clinical.